Old Weapons and New: How We Can Win The Fight Against Malaria
A fundamental axiom of war is "know your enemy". One of our deadliest enemies is malaria. Malaria kills almost half a million people, mainly children, every year. Malaria knows us all too well: it manipulates us, it resists our drugs, and it deceives our immune system.

With the aid of science, however, we can hope to understand malaria just as well as it knows us. The life-cycle of malaria is complicated. But to understand it helps us identify chinks in malaria's armour.
In this post, I'll trace the life-cycle of malaria and explain how we can use that knowledge to develop new weapons against malaria. I'll focus mainly on vaccine strategies, but I will discuss drugs, bednets and other interventions too. By the end I hope I'll have shown that the fight against malaria is one we can win, and one where we can all make an important contribution.
Know Your Enemy: Malaria
Malaria is neither virus nor bacteria. It is a large, single-celled organism, categorised as a parasite, more closely related to ourselves than to bacteria or viruses. It spreads via mosquitoes. A female mosquito, seeking blood for her eggs, will land on you and spit a soup of salivary juices into your skin prior to sucking your blood. If she harbours malaria she will also spit 50 or so malaria parasites into your skin.
These malaria parasites actively wriggle about until they find a tiny blood vessel. They then corkscrew into that blood vessel and swoosh off down your blood stream. The parasites are transported everywhere in your body but ultimately they stick in your liver because of proteins on their surface that bind to your liver cells.
The malaria parasites quickly enter your liver cells and spend about a week growing and multiplying. Each parasite becomes thousands. Your infected liver cells begin to bulge ominously. Finally they break into little packages containing a hundred or so parasites.
These packages swoosh away in the blood-stream until they become jammed in the narrow blood vessels of your lungs. The packages burst and blood-stage parasites spill out. These blood-stage parasites must find a red blood cell fast and invade it or they will die. Luckily for the parasite there are thousands of red blood cells all around it. They bind, invade, and begin replicating inside. Cycle after cycle of invasion occurs, entering red blood cells, replicating, bursting out, and invading fresh cells.
It is now that symptoms begin to show and dangerous illness can occur. The growth of the parasite is exponential and it is not uncommon for 5% of blood cells to become infected. Your body can mount such a strong immune response to this that in many cases it leads to death or to coma. Severe anaemia is also a major consequence of malaria as your body destroys millions of infected and uninfected red blood cells in an attempt to control the infection.
This harms you, the human, but the parasite must get back into the mosquito if its life-cycle is to continue. Most of blood-stage parasites are asexual; however, some male and female forms are also produced. These sexual stages are taken up in your blood when a female mosquito lands on you and bites you. Inside the mosquito, the male and female parasites find each other, fuse, and burrow into the mosquito's gut. For three weeks they develop, replicating into the thousands. Finally they explode into the mosquito's bodily fluids, swimming until they reach her salivary glands. Here they wait, ready to be spat into the next victim of a mosquito taking her blood meal.
Long story short, there are three stages in the malarial life-cycle:
- the pre-liver stage, where the parasite is injected by a mosquito into your skin and travels to your liver;
- the blood stage, where the parasite undergoes round after round of replication inside your red blood cells;
- the mosquito stage, where the sexual forms of the parasite are taken up by another mosquito and invade the salivary glands ready to infect another human.
Here is a life-cycle diagram from the CDC which illustrates it nicely.
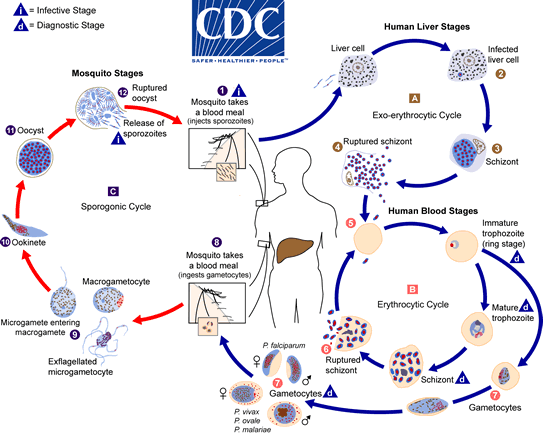
What weaponry do we possess, and what new weapons can we develop using this knowledge?
Weapon 1: Vaccines
A good vaccine against malaria would be an extraordinarily powerful weapon. The only human disease ever to be eradicated – smallpox – was eradicated using a vaccine. Polio will likely be the second human disease ever eradicated, also by vaccination. Vaccines are the most cost-effective health interventions ever developed. It is worth describing how they work.
A vaccine educates your immune system to recognise and defeat an infectious organism before you ever encounter it. If you get chickenpox for the first time, your immune system will be slow to combat it at first, but then you will develop antibodies and other immune weaponry that specifically and effectively targets it. Next time you get chickenpox your immune system recognises it and rapidly deploys the same antibodies that defeated it last time, except much more quickly than before. Chickenpox is defeated before you even notice you've got it.
With diseases like smallpox, polio and malaria, it's really best not to get the disease even once. Your first encounter could well be your last. A vaccine is an artificial way to mimic infection and induce your body to prepare antibodies and other immune weaponry before you encounter the disease.
There are various ways to do this. With smallpox, a related virus called vaccinia is used to vaccinate you. (The name is not coincidental: vaccinia was the world's first vaccine). Vaccinia and smallpox look very similar to your immune system: if your immune system recognises vaccinia, then it will recognise smallpox. Vaccinia is completely safe for humans, so it is a great way to train your immune system to recognise and defeat smallpox.
An alternative to using a closely related but harmless version of the disease is to use the disease itself, killed or weakened in some way. Polio is an example of this: it is either killed outright, or a mostly harmless (mutated form)[http://www.who.int/biologicals/areas/vaccines/polio/opv/en/] is used. Then you either eat it (for instance, on a sugar cube) or it is injected. Thus rendered harmless, the polio virus can't cripple you or damage you; but you can still develop an immune response against it. When polio really infects you, your immune system will be ready to defeat it.
There are two main weapons of immunity a vaccine can induce: antibodies and T-cells. Most vaccines, polio and smallpox vaccines being examples, act by generation of antibodies. Antibodies are Y-shaped proteins that bind to specific bits of the disease-causing organism. They often work by preventing that bit of the disease organism's machinery from carrying out its function (for instance, blocking it from invading your cells). T-cells, meanwhile, recognise that your cells have been invaded and kill them. Antibodies are best at stopping a disease-causing organism before it enters a cell; but T-cells are best at destroying cells that have become infected.
Malaria vaccine development has been going on for a long time. A key breakthrough came in the 1960s when researchers in New York blasted malaria-infected mosquitoes with radiation. When these radiation-blasted mosquitoes were allowed to feed on human volunteers they went on to develop almost complete immunity to malaria. The radiation damages the parasite's DNA just enough to allow it to invade the liver as usual, but not develop beyond that point. The immune system thus develops a very powerful T-cell response against the parasite. When you become infected with malaria as normal those T-cells can track down and destroy every single infected liver cell before you get blood-stage malaria. You never get sick.
The problem with this approach is that a lot of mosquito bites are required. To avoid this a US company called Sanaria (meaning 'healthy air' in contrast to the 'bad air' for which malaria is named) have set up a human assembly line of mosquito-dissecters, removing the parasites from mosquitoes and purifying them for direct injection (after neutralising radiation treatment) into humans.
Other groups are developing an alternative to radiation by genetically altering parasites so that they can't develop beyond the liver stage; the effect is the same or even stronger.
Unfortunately none of these ingenious whole-parasite vaccines are likely to be deployed where they are most needed, in tropical zones. Whole-parasite vaccines require storage and transport in liquid nitrogen, at –210°C while many rural areas in sub-Saharan Africa are infamous for their lack of functional freezers. Whole parasite vaccines will also require no less than five injections into a vein to work, a tough sell: most healthy people dislike having just one intravenous injection so persuading people to come back for five may be a difficult task.
Nevertheless, whole-parasite vaccines show that a good malaria vaccine is possible. It is a question of making one that is practical. A vaccine that has gone a long way towards achieving this is Mosquirix by GlaxoSmithKline. 30 years in the making, Mosquitrix is on the verge of becoming the world's first licensed malaria vaccine, having obtained a positive opinion from the European Medicines Agency last year.
Instead of using a whole parasite, in Mosquirix a single protein from the surface of the parasite is used. It is called circumsporozoite protein, or CSP for short. Mosquirix generates huge antibody responses against CSP and since CSP is one of the proteins the parasite uses to invade the liver, these antibodies can block the parasite from invading your liver cells. If the parasites do manage to invade your liver cells, there may be enough CSP inside them that T-cells can come along, recognise that the liver cell has been invaded, and destroy it. However this mechanism is a minor contributor; Mosquirix protects mainly by through antibodies.
This vaccine is about 35% effective, meaning that Mosquirix reduces the number of cases of clinical malaria in children by about 35% over a two year period. This is very low: for comparison, the polio vaccine is about 95% effective. In areas where it is really needed, Mosquirix with its 35% efficacy level could still have a positive impact. Whether it is cost-effective or not depends on what GlaxoSmithKline intends to sell it for; this vaccine will likely, however, remain less cost-effective than bednets.
The global health community is aiming for a malaria vaccine that is 75% effective and to make it by 2030. This will be difficult but perhaps not impossible. The most promising strategy at present is to combine vaccines that work at different stages of the parasite life-cycle: the pre-blood/liver stage, the blood-stage, and the mosquito stage.
One option could be combining a vaccine like Mosquirix with one called TRAP. People vaccinated with TRAP generate strong T-cell responses. These T-cells hunt out and destroy malaria-infected liver cells.
The TRAP vaccine is about 21% effective, lower than Mosquirix. One reason for the low efficacy is that hunting for malaria-infected liver cells is like looking for a needle in a haystack. There may be as few as twenty liver cells infected with malaria in a typical case. Since each liver has hundreds of billions of cells, you can imagine how overworked these poor T-cells must be. If just one infected liver cell escapes detection then all that hard work counts for nothing. It takes only one infected liver cell to give you full-blown malaria.
If TRAP were combined with a Mosquirix-style vaccine, antibodies against CSP could reduce the number of parasites reaching the liver and anti-TRAP T-cells could mop up the rest of the infection. A recent study suggests this strategy can work, in mice at least.
Ultimately this strategy will be extended to include blood-stage vaccines. There are several in development based on blood-stage proteins like EMP1, MSP1 and RH5.
Malaria of course does not make the task easy for us. The EMP1 protein is coded for by a family of over 60 genes. Each of these genes can be cycled through in turn during a single malaria infection. By the time your immune system has learned to recognise the first version of EMP1, the parasite no longer uses it and can escape destruction.
With other proteins, like MSP1, the problem is there is so much variation between malaria strains scientists struggle to achieve a vaccine that recognises every strain. RH5 is a good blood-stage vaccine candidate for the reason that it doesn't vary much between strains. Combining it with pre-blood stage vaccines should enhance protection even further.
The third and final type of vaccine targets the mosquito stage, which is fairly remarkable. Normally antibodies can only act within your body. But mosquitoes draw up antibodies along with your blood, so if you are immunised with a vaccine targetting the sexual stages of the parasite, they can act in the mosquito against the malaria parasite. This can prevent the parasite from developing into human-infectious forms.
Often these mosquito-targeting vaccines are called 'transmission blocking vaccines' because, if they work, they interrupt the life-cycle of malaria. They are also called 'altruistic vaccines' because they confer no immediate benefit on the individual who is immunised: that person will still suffer malaria symptoms if they are infected. But your community will benefit; if enough people are vaccinated, malaria rates will drop, contributing to the local elimination or even global eradication of the disease.
An exciting new extension of this idea is the genetic modification of mosquitoes so they themselves make anti-malarial antibodies. It prevents the mosquitoes from transmitting malaria. If we released sufficient numbers of these GM mosquitoes into the wild, they could well reduce malaria transmission significantly and potentially be very cost effective. As with all proposed releases of genetically modified organisms, however, the idea is controversial.
Weapon 2: Drug Treatement
Vaccines are weapons in development, but drugs are weapons on the front line. Like vaccines, drugs normally act only against a specific stage of the malaria life-cycle, and usually it is the blood-stage.
There is only one drug available that targets the liver-stage parasite: primaquine. Mass drug administration of primaquine is currently impossible because as well as destroying infected liver cells it also destroys red blood cells in people with a G6PD deficiency. Rapid diagnostic tests exist to identify who will react this way and who won't, but these tests aren't currently cheap and reliable enough to permit mass-administration of primaquine.
Chloroquine, mefloquine and the Nobel prize-winning artemisinin are some of the drugs that work against the blood stage. They work well. That is, for a time. Sooner or later resistance develops to every anti-malarial drug, and sometimes it takes as little as five years for resistance to emerge.
This is frightening. We rely heavily on drugs to fight the disease, both to stop people getting ill and to cure those who contract the infection. The WHO currently recommends combining drugs, like artemisinin with mefloquine. The idea is to make it more difficult for the parasite to develop resistance to one of the drugs. It is only a matter of time before these swords become blunt, however. We need to forge new swords and fast.
In war, the friend of my enemy is my enemy: we should also attack the ally of malaria, the mosquito. One approach involves another type of genetically-modified mosquito, this time possessing a gene which renders female mosquitoes infertile. The GM mosquitoes also possess a gene editing system which causes the infertility gene to spread. In small greenhouse trials when these infertility gene-carrying mosquitoes are released within a normal population, four generations is all it takes for the infertility gene to spread by 50%. However, the ecological effects of wiping out mosquitoes and of releasing this gene-drive technology in the wild are unknown, which many see as good reasons to hesitate about using this technology.
Weapon 3: Bednets

Our mightiest anti-malarial weapon has been left until last. The long-lasting insecticide-treated bednet takes pride of place in our arsenal. Since 2000 malaria infection rates have halved in Africa and nearly 70% of this reduction is due to insecticide-treated bednets. New weapons – new drugs, new vaccines – would be very useful, and might be essential to eradicate malaria. But the bednet will always remain one of our central weapons in our war against malaria; that is certain.
There is one other weapon which is worth mentioning: money. This war will be a long one. As with all extended conflicts, victory will come down to how much money we put up for the fight. It is astonishing and revealing that our most powerful anti-malarial weapon, the bednet, is also so inexpensive. Effective altruists know this very well: it is why the Against Malaria Foundation, which distributes insecticide-treated bednets, is so highly recommended by Giving What We Can and Givewell.
At the same time, this fact demonstrates that global institutions have failed. Bednet distribution ought already to be fully-funded, rendering effective altruists unable to spend their money on them. The yearly shortfall in malaria funding is staggering – in the billions – and this is especially tragic given how relatively easy it is to treat and prevent the disease and how much good it would do. Effective altruists, as individuals, have stood up where institutions have fallen down. The battle against malaria is part of a larger war against unnecessary suffering: long may we continue to fight it by giving effectively, giving much, and spreading the word far and wide.