Summary
The Against Malaria Foundation (AMF) has been one of our highly recommended charities for several years now. Our colleagues at Givewell have published an extensive update recently that was generally very favourable and they continue to rank among Givewell’s top charities. You can read more on Givewell’s AMF page. In this blog post, we give you an update about their efforts that we feel complement Givewell’s report. You can find more general information about AMF on our website. In our opinion, AMF continues to be one of the most effective charities in the world.
Recent Scientific findings that relate to AMF’s effectiveness
Malaria Disease interactions
Does Malaria increase HIV infections?
There is some evidence that malaria increases HIV transmission[1]. In 2006, a paper published in Science showed that people who are HIV positive have shown a spike in HIV viral load during a malaria fever episode[2]. They found that, a malaria fever can increase HIV viral load by almost one log (10 times) - and stay at an increased level for a duration of up to 6 weeks[3]. A recent systematic review and meta-analysis found that acute malaria increases HIV viral load by only 0·67 log10 (see [4]).
But does this increase in viral load cause increases the risk of transmitting HIV? According to a recent review, this seems to be the case: malaria may increase HIV transmission in communities with high HIV prevalence, but has no effect in areas with low HIV prevalence[5].
How large is the increase? Early modelling data suggested that in Kenya, since 1980, this disease interaction may have caused 8,500 HIV additional infections (and about 1 million extra malaria episodes due to HIV - this co-infection might also have facilitated the geographic expansion of malaria to areas where HIV prevalence is high)[6]. Another study showed that individuals who live in areas with high malaria parasite rate have about twice the risk of being HIV positive compared with individuals who live in areas with low malaria parasite rate, after controlling for important socioeconomic and biological factors[7]. Surprisingly, the cause of the high HIV prevalence in sub-Saharan Africa is not completely understood[8]. One of the unsolved question was why HIV spread so rapidly in Sub Saharan Africa, even though there is a very low probability of HIV transmission through heterosexual sex, which is commonly assumed to be the main driver of HIV transmission (estimated to be ~ 1/300 per coital act in low income countries, where ulcerative STIs are more prevalent[9])[10]. Some researchers have suggested that it is high HIV-1 plasma viral loads in sub-Saharan Africa, partly because of high rates of coinfection, may have been one of the drivers of the “explosive” epidemics seen in that region[11].
A very recent study[12] modelled the coinfection of HIV with malaria and other diseases such as schistosomiasis. Based on their findings, they argue against the notion that explosive HIV epidemics in sub-Saharan Africa has been driven by higher community viral load due to coinfections. They concluded that duration of coinfection is too short and/or the viral load elevation is too modest to reduce HIV-1 transmission or the duration of asymptomatic HIV-1 infection substantially. The authors estimate that for every 100 HIV-infected people, each suffering 1 episode of malaria infection, there are only 0.4 (2.7th– 97.5th percentile [95% confidence intervals]: 0.0–2.0) to 2.4 (0.0–21.0[13]) (depending on whether there are multiple infections per year) additional HIV-1 transmission events attributable to HIV-Malaria coinfection. For lymphatic filariasis, which bednets might also offer some protection from (see below), it is 13.3 (0.3–89.2).
In sum, the research on HIV malaria interactions is inconclusive and more research is needed to investigate these interactions. However, independent of the effect size, and whether the coinfections are a/the main driver of the HIV epidemic in subsaharan Africa or not, some authors have argued even a small increase in transmission risk from these cofactors generates large numbers of new HIV infections. Thus, for the present case of estimating the effectiveness of bed net distribution, we believe that there is good reason to assume that net distributions are even more cost-effective than previously assumed - we just do not know by how much at this point. In any case, the full economic cost-benefit analysis includes both the positive effects of malaria prevention, which is high already, plus any additional positive effects on top of this that one gets ‘for free’, even if future research estimate these effects to be small. For people who particularly value disability and morbidity during adulthood as opposed to early childhood morbidity[14], this might be a reason for the Against Malaria Foundation becoming more attractive. Some researchers have argued cofactor-related interventions are only hesitantly included into African HIV prevention strategies[15] and that among policy makers and donors, however, there is still some resistance to investing in bed nets (and other preventative health interventions), to reduce new HIV infections.[16]
Finally, two recent studies suggest that independent of whether and by how much HIV transmission risk is increased, coinfections might have implications for distribution of malaria nets to people infected with HIV. One study suggested that people with HIV that were provided with bednets had slower progression to AIDS[17] and another study showed that people with HIV who were provided nets and water filters delayed initiation of antiretroviral therapy, potentially due to this slower progression to AIDS[18]. The later study estimated this intervention to be highly cost effective: when combining the benefits due delayed initiation of antiretroviral therapy with other health benefits due to fewer malaria cases, this interventions costs US$99 per disability-adjusted life year (DALY) averted (with net cost savings for the health system). Another recent study found that almost all the HIV positive patients in a sample with malaria infection were anemic[19]. But can net distribution reduce the morbidity of people who are infected with HIV? A recent review concluded the data were very limited regarding the impact of net provision and/or use on malaria morbidity reduction in people with HIV. The quality of the four studies they reviewed was rated as “medium.”, the overall quality of evidence was rated as “fair”, and and the impact of net provision on malaria morbidity was rated as “uncertain”[20]. More research needs to be done to investigate this interaction.
Malaria and Anemia
1.62 billion people have anemia. 41.8% of all pregnant women have anemia, with the majority living in Africa: 17 million pregnant women are known to be affected[21][22].
The consequences of malaria-induced anemia in pregnancy has been well established and the impact of distribution of bednets to pregnant women are described in a 2006 Cochrane review[23]. The evidence shows that sleeping under nets compared to not sleeping under nets increased mean birth weight by 55 g, reduced low birth weight by 23%, and reduced miscarriages/stillbirths by 33% in the first few pregnancies. This strongly suggests that it is a good idea for pregnant women to sleep under nets in Africa[24]. However, as of 2010, insecticide-treated nets were used during pregnancy for only 10·5 million of 26·9 million births across 37 countries[25]. Finally, a recent paper showed that there was also a significant association between anemia and asymptomatic malaria among pregnant women.
Another affected group are HIV positive patients: a recent study found that almost all the people in a sample with malaria infection were anemic[26]. Few studies have looked at the effect of malaria on anemia in non-pregnant, non HIV-infected adults. One study in Cameroon however suggested that in adult patients with fever, malaria parasitaemia contributes to anaemia and that this is of public health impact[27]. This improves the case that reduction of malaria through net distributions also decreases incidences of anemia.
Malaria and Lymphatic filariasis
Synergistic effects of using nets to prevent both malaria and lymphatic filariasis have been investigated for a long time[28],[29], as both diseases are transmitted by Anopheles mosquitoes (see Table 1 (adapted from[30]). A recent paper calls for integrated vector management and concludes that the available data show that the use of treated or untreated bed nets and indoor residual spraying for malaria control concomitantly also reduced lymphatic filariasis[31]. For this reason, Nigeria has recently launched the first nationwide lymphatic filariasis and malaria co-implementation plan, based on distribution of both preventive chemotherapy medication and long-lasting insecticidal nets[32].
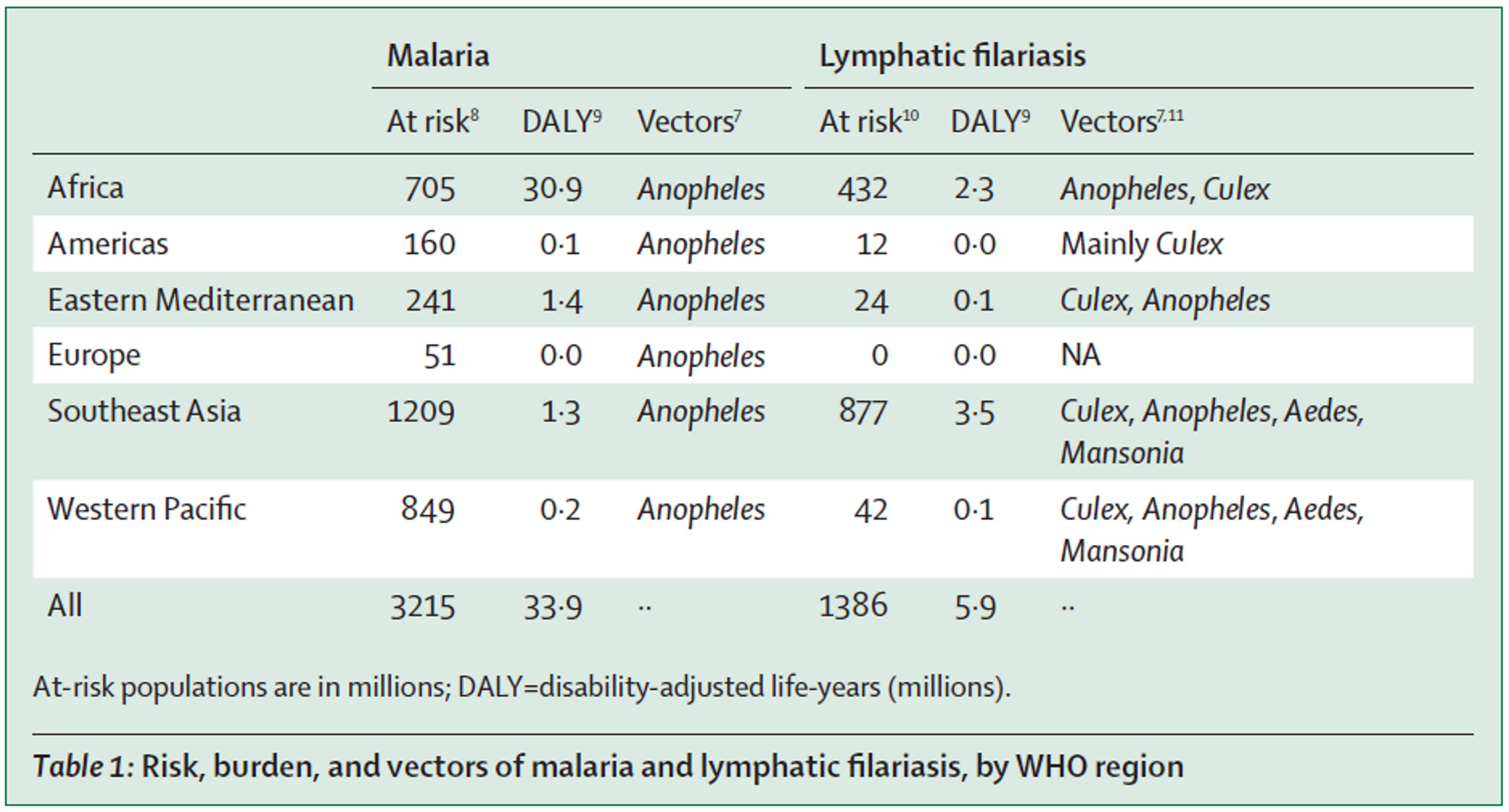
AMF currently plans to carry out all of their future net distributions in Malawi and the DRC. The DRC has one of the highest prevalences of lymphatic filariasis[33] and no mass drug administration against it has been carried out hitherto[34].
Malawi also has high prevalence of lymphatic filariasis and and mass drug administration coverage against lymphatic filariasis is incomplete (about 80%)[35]. One recent study suggests that malaria interventions such as bednet distributions likely have also affected LF transmission[36].
Studies show that treating lymphatic filariasis coinfection in HIV‐positive patients significantly reduced their viral load[37]. A modelling study estimated that this might increase transmission risk and that for every 100 HIV-infected people, each suffering from lymphatic filariasis, there are 13.3 (0.3–89.2) additional HIV-1 transmission events attributable to HIV-lymphatic filariasis coinfection[38].
Malaria and Blood pressure
One recent study suggests that malaria during pregnancy might affect blood pressure in children and that this might may contribute to the African burden of hypertension, which is higher than in developed countries[39].
Prevalence of Malaria
A recent paper[40] used a more sensitive test for asymptomatic malaria and showed that malaria is more common than previously thought: the prevalence of malaria in the study’s population was underestimated by 8%. Asymptomatic malaria carriers influence the transmission potential of the disease [41]. This suggest that the malaria burden might be underestimated.
Another review has recently concluded that the morbidity burden of Malaria has been underestimated in particular with regards to adult morbidity[42].
Malaria and climate change
A recent study suggested that increasing temperatures due to climate change might mean that future climate becomes more suitable for malaria transmission in the tropical highland regions[43].
Insecticide resistance
Evolution of resistance to insecticides continue to worry researchers: resistance in mosquitoes has been reported in 53 of 65 reporting countries around the world since 2010[44]. However, a recent meta-analysis concluded that ITNs are still more effective than nets that are not treated with insecticides regardless of resistance[45] and distributing nets remains a cost-effective health intervention, even in areas with strong insecticide resistance[46].
Does providing free bednets increase the number of people sleeping under bednets?
It has been hypothesized that people who purchase nets will use them more than those who receive them for free and thus that free bednet distributions are not as good as selling them.
A recent Cochrane meta analysis[47] reviewed the evidence on this and suggests that there is probably little or no difference in net use among those who receive a free net compared to those who pay for one. They suggest that providing free insecticide-treated bednets probably increases the number of people who own bednets compared to providing subsidized bednets or bednets offered at full market price[48]. Another study showed that children who receive their nets through NGOs or government likely receive some education about bednets and are more likely to use them in comparison to those children who receive nets that are bought privately through private health centers, market, shops and street vendors[49].
Two recent studies tried to answer the question of whether providing free bednets affects private net sales negatively. One study showed 34 per cent monthly decline immediately after a free bednet distribution campaign compared to ‘normal’ sales[50]. They found that after 6 months, the total unsold nets reached 45 percent of normal sales, or 347 000 nets nationwide in Tanzania. They caution that free campaigns can hinder private net sales much more than previously observed in recent experimental trials and thus can cause trouble with continuous coverage delivery channels of bednets through private sales. In contrast, another study also in Tanzania, showed distribution of free insecticide-treated nets do not interfere with continuous net distribution[51]. The authors argue that the discrepancy between the two studies is likely because they used to different data from different time points. We cannot conclude much from only two studies with opposite findings, but whether free net distributions displace private net sales should be subject of further study and observation.
Net durability
Two recent papers challenge the previously held assumption that net durability is about 3 years.
Insecticide treated nets with holes in them are less effective at protecting people from malaria than intact nets[52]. In line with this, monitoring of durability of nets in Rwanda showed greater than anticipated bednet loss, associated with poor fabric integrity, during year two of a three year LLIN distribution-replacement cycle. The proportion of the remaining nets in need of replacement, after two years, was large enough to suggest that the intervention would lose impact during year three of the distribution-replacement cycle[53] and this might be one of the reasons for resurgence of malaria in Rwanda in 2009, following a 2006 under-five, country-wide bed net campaign. Similarly, another study suggests that the bednet in distribution conducted every three years, which is a key intervention of Benin’s malaria control strategy, is insufficient and a two-year serviceable life for the current LLIN intervention in Benin would be a more realistic program assumption.[54]
Another recent analysis suggested that because of previously unaccounted factors such as uneven distribution of nets and rapid net loss, it will require substantially more nets than currently assumed in order to meet international coverage targets[55]. All this suggest that more bednets are probably needed than previously assumed, which is favourable for AMF and their part in trying to close this gap.
Misuse of Malaria nets
A recent article in the New York Times[56] “claimed countless [people in Africa] are not using their mosquito nets as global health experts have intended”, most often for fishing, which causes harm to fish stock due to the nets insecticides and fine gauges. Givewell has already commented on this piece[57], summarizing the data coming from actual monitoring as opposed to self-reported surveys that might be biased. The monitoring data is generally in line with this.
AMF[58] and other organisations[59] have commented on this piece. AMF takes this concern seriously and takes steps to prevent net misuse such as malaria education, pre-distribution surveys to determine precise net needs, post-distribution check-ups to ensure nets are being used as intended every 6-months. Givewell has reported on AMFs monitoring data in Ntcheu[60]: this data shows that 81% of nets were still hung 24 months post distribution and only 15% were in a poor condition (the net has more than 10 small holes or has one or more large holes) and 8% worn out (quantified as having multiple large holes and the LLIN is unrepairable, such that it would not provide protection against mosquitos). AMF found that 77% LLINs were in "very good" or "OK" condition at 24 months. The nets decay was faster in Balaka, where 70% of nets were in "very good" condition at 6-months (and 25% in "OK" condition), compared with 99% in "very good" condition at 6-months in Ntcheu[61].
We could only find one study that quantified the extent of bednet use in fishing: a small survey study with 196 respondents in seven villages surrounding a Lake Tanganyika reported that 87% of households surveyed have used a mosquito bed net for fishing at some point. However, another much more comprehensive analysis of 14 surveys in several countries with 14,196 households showed that that the overwhelming majority of nets were used for malaria prevention, and only 255 nets were repurposed (which make up less than 1% overall). Furthermore, the majority of the repurposed nets were already considered too torn, indicating they had already served out their useful life for malaria prevention[62]. The authors conclude that national programmes and donor agencies should remain confident in the appropriate use of bednets.
Mass drug administration against Malaria
Mass drug administration (MDA) of ivermectin to humans for control and elimination of filarial parasites can kill biting malaria vectors and lead to Plasmodium transmission reduction[63],[64]. A recent review suggested that there is good evidence for the significant reduction in malaria transmission following single ivermectin MDAs across diverse field sites[65] and other highlight that this should be seriously considered as a new tool against Malaria as nets do little to prevent outdoor transmission[66]. However, MDA is not going to replace bednet distribution, but rather be used in addition to it and thus this does not decrease AMF’s effectiveness.
Economic effects
Givewell had previously cautiously suggested[67] that a 2010 study from Bleakley et al.[68] that looked malaria eradication programmes in the United States in the 1920s and in Latin America in the 1950s, and concluded that cross-regional differences in malaria might explain differences in income, with people in regions with less malaria had higher incomes.
We found three more studies investigating economic benefits of campaigns aimed to reduce malaria. One study found that malaria eradication in India lead to modest increases in household incomes for prime age men, but not on education[69]. Another study found suggestive evidence that malaria eradication in Uganda increased income levels.
The third study calculated the probability that elimination would be cost-saving over 50 years in 5 different sites. Their estimates ranged from 0% to 42%, with only one site achieving cost-savings. They suggest elimination might still be a worthy investment, but financial savings should not be a primary rationale for elimination. However, they call for more robust research into the effects of malaria elimination[70].
The third more recent study looked specifically at the use of insecticide-treated Bednets in 18 African countries for children under 5 years of age. They found that on an annual basis, nets can result in a 6.2 percent reduction in outpatient costs, a 6.6 percent reduction in inpatient treatment costs, a 6.3 percent reduction in productivity losses, and a 22.6 percent reduction in disability adjusted life years (DALY)[71].
Educational benefits
A recent study examined the effects on the Global Fund’s malaria control programs on the educational benefits to primary schoolchildren in Sub-Saharan Africa[72]. Using a quasi-experimental approach, they found that in a large majority of countries (14 of 22), the program led to substantial increases in years of schooling and grade level as well as reductions in schooling delay.
Updates on Room for additional funding
Givewell estimates that total cost per distributed bednet by AMF is about $5.30 -$7.50 depending on the country. AMF estimates that it is only $4.80, because it does not account for pro bono services. This number is much lower than $5.80 (calculated on a similar basis, and therefore comparable to the $4.80 figure), which is what it costs other organisations to distribute a net.
The recent Givewell report on AMF estimates that AMF has room for up to $25 million of additional funding to support its activities in 2015 and that they hope to contribute about $10 million to them.
Success in large scale distribution
Givewell was hesitant to recommend more funding due to AMF’s lack of track record of successfully completing large-scale net distributions (>100k nets) with partners other than Concern Universal in Malawi. However, now AMF reports that it has succeeded in completing its largest distribution of nets to date in in the DRC with a different partner: 676,000 nets were successfully distributed in late 2014 according to AMF and their distributor, though photo and video evidence of the distribution is still unavailable[73]. This increases our confidence that AMF is able to successfully complete large scale distributions.
Flood-related emergency need
In Malawi, a large scale distribution of 396,000 nets was planned in in January-February 2015[74]. Incidentally, during this time, Malawi has experienced the worst floods in 50 years with at least 174,000 people have been displaced[75]. AMF reports that they allocated a further 150,000 LLINs to support an immediate flood-related emergency need in Malawi[76], because there is increased malaria prevalence after heavy rains. Emergency responses are often viewed as more costly and less effective than preventive health intervention. However, in the case of emergency bednet distribution, the benefits are likely to be twofold: above and beyond the positive effect of the emergency response, there are preventative effects of the long lasting insecticide treated nets that last beyond the emergency response. Thus, emergency response net distributions can be seen as an additional benefit on top of preventative effects. We are not aware that this aspect has been taken into account when calculating room for more funding calculations in the past. Based on this one might argue that AMF is in a unique position to be deserving of building up larger reserves to avoid shortpasses. Givewell had in the past temporarily suspended their recommendation of AMF[77], because they believed that AMF had too many reserves, while we recommended AMF throughout[78].
Long-term view on room for more funding
We have previously reported that Schistosomiasis Control Initiative, one of our other top recommended charities, has a lot of room for more funding, if it were to scale up its operations, given the scale of the problem of parasitic infections and their unique expertise in conducting mass drug administrations. We assumed that SCI’s expertise could be transferred to conduct mass drug administrations against other diseases than worm infections such as lymphatic filariasis.
With AMF, the situation is slightly different, because their core operation is only involved in bed net distribution and we are not sure about how far their expertise could be used in other areas.
However, there is still a massive gap in bednets needed in Africa. We now present some estimations of the net gap.
Programmatic gap analysis of bednets for the short-term need in nets
The WHO’s ‘Roll Back Malaria’ Harmonization Working group has recently updated their estimates of the net gap for 2015[79]. They estimate that there still exists a gap of about 49 Million nets in Africa, which translates to a funding gap of about 315 Million dollars for 2015 (for our calculation see our excel spreadsheet[80] - and Table 2). Thus, we think there is room for more funding for AMF in the near term. Givewell too, reports that AMF could scale up its operations if it is successful in finalizing large scale bednet distributions. These calculations provide an indicator for a rough estimate of an upper bound of what AMF could conceivably spend in the near-term future.
|
LLIN commodity Gaps
|
2015
|
2016
|
2017
| |
Need
|
255,621,533.00
|
185,448,267.00
|
216,883,819.00
| |
Financed
|
206,044,404.00
|
112,323,391.00
|
54,470,603.00
| |
Gap
|
49,577,128.00
|
73,124,876.00
|
162,413,216.00
| |
LLIN Funding Gap in $ (net gap multiplied by average of Givewell's net cost estimate: $6,39)
|
2015
|
2016
|
2017
| |
Need
|
$1,633,421,595.87
|
$1,185,014,426.13
|
$1,385,887,603.41
| |
Financed
|
$1,316,623,741.56
|
$717,746,468.49
|
$348,067,153.17
| |
Gap
|
$316,797,847.92
|
$467,267,957.64
|
$1,037,820,450.24
|
Table 2: Overall funding gap for bednets and an estimate of its associated costs (based on[81])
Some people might be surprised that the number in needed nets in 2015 is ‘only’ 49 million nets. This is because this this gap is the ‘programmatic gap’, defined as the nets needed to cover the gap between the current coverage and the planned targets that are not based on the actual net need, but only on what level of bed net distribution was deemed realistic at a certain point. For instance, this assumes that for every net distributed to malaria endemic areas, 1.8-2.0 are covered and 80% of the population should be covered[82]. This means that even though now many people live in a household with an insecticide treated net, which might offer some protection even if they are not the family member sleeping under it (see Figure 1 and Figure 2), not every person who should ideally be sleeping under a net actually is sleeping under a net (children under five and pregnant women are more likely to sleep under nets, but ideally adults and adolescents should also sleep under nets). Thus, this gap analysis is likely to be an underestimate of the true need for nets. What is the true need for nets? A recent WHO report shows the difference between households who have at least one net and households with enough nets for all occupants.
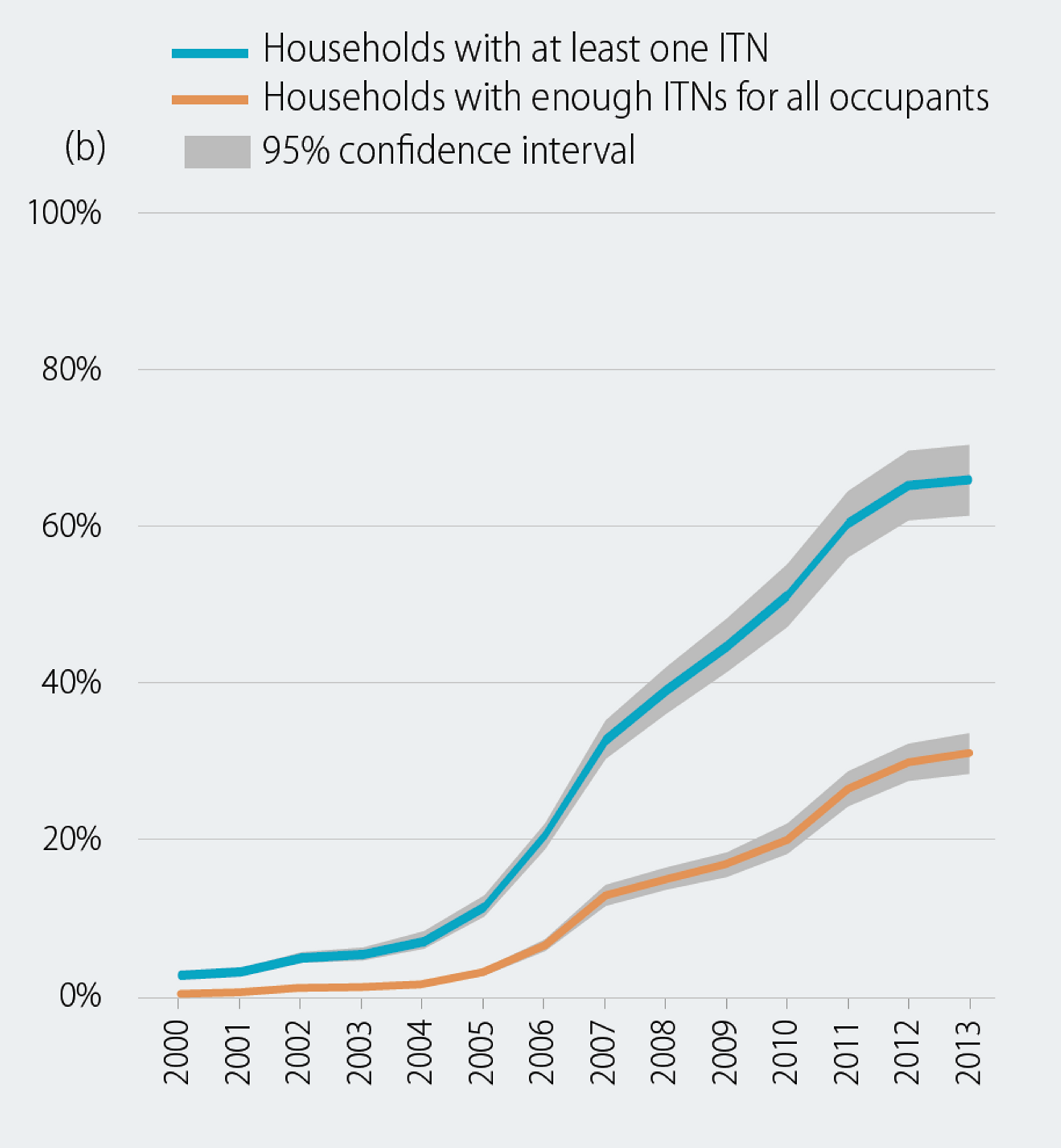
Figure 2 adapted from[83]: Proportion of population with access to an ITN and proportion sleeping under an ITN, b) Proportion of households with at least one ITN and proportion of households with enough ITNs for all persons, sub-Saharan Africa, 2000–2013
A recent paper independently arrives at a very similar conclusion: they argue that the previously held assumption that the people do not use nets because the importance of sleeping under a net is not communicated clearly enough is false. In fact, there are not enough nets within the households for everyone to sleep under[84].
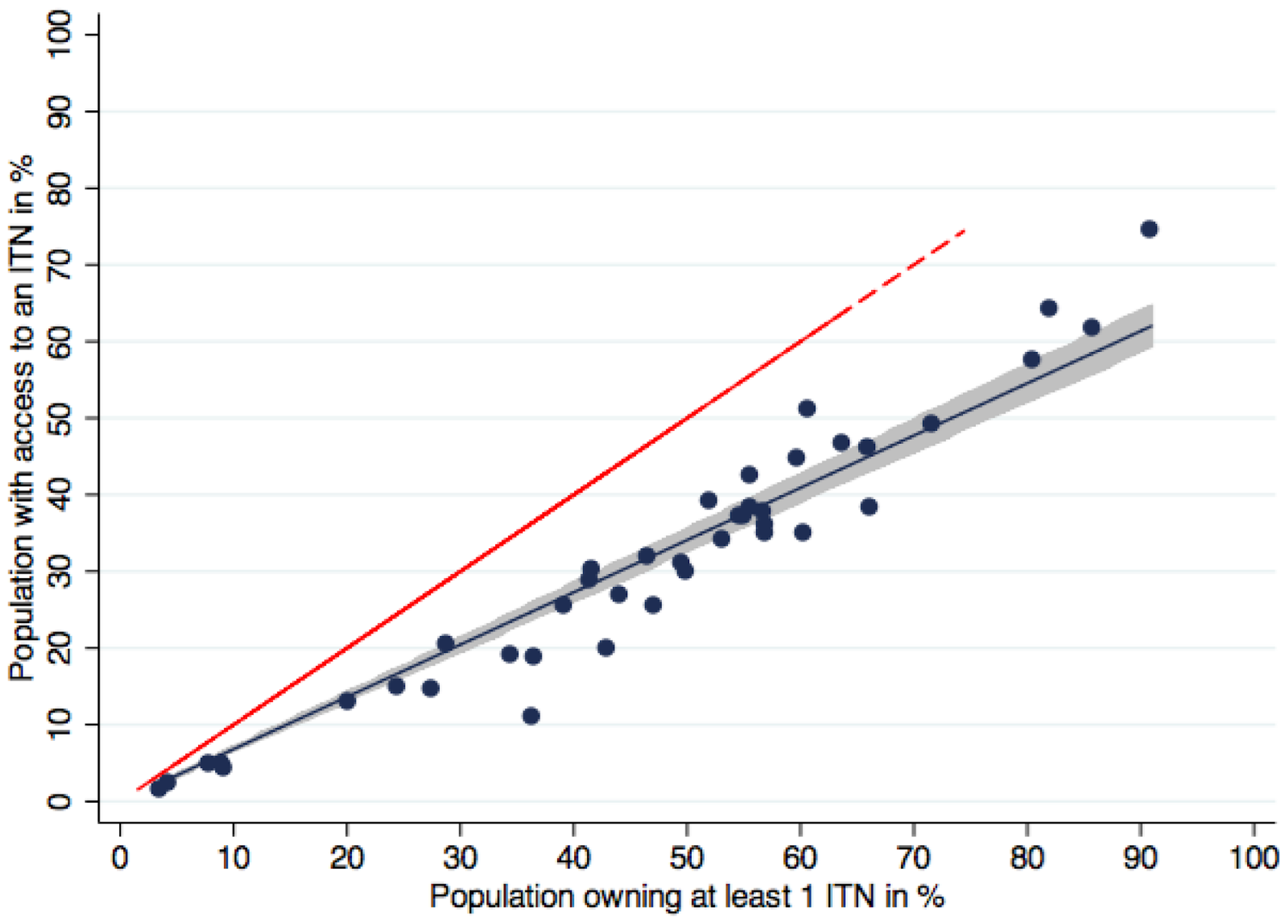
Figure 2 adapted from[85]. Population with access to an ITN within the household compared to ownership of at least one ITN.
Blue dots represent the data points for data sets, the blue line the regression function (fitted values). Shaded area is the 95% confidence interval of the fitted values of population with access to an ITN within the household. Red dashed line represents the equity line where ownership is equal to access. On average, population access was 32% lower than household ownership.
Net usage in Africa has developed rapidly (see Figure 3). In 2005, net coverage was very low, with only six countries achieving coverage levels greater than 20% (Figure 3.2), and coverage remained low for the next few years (see Figure 3).
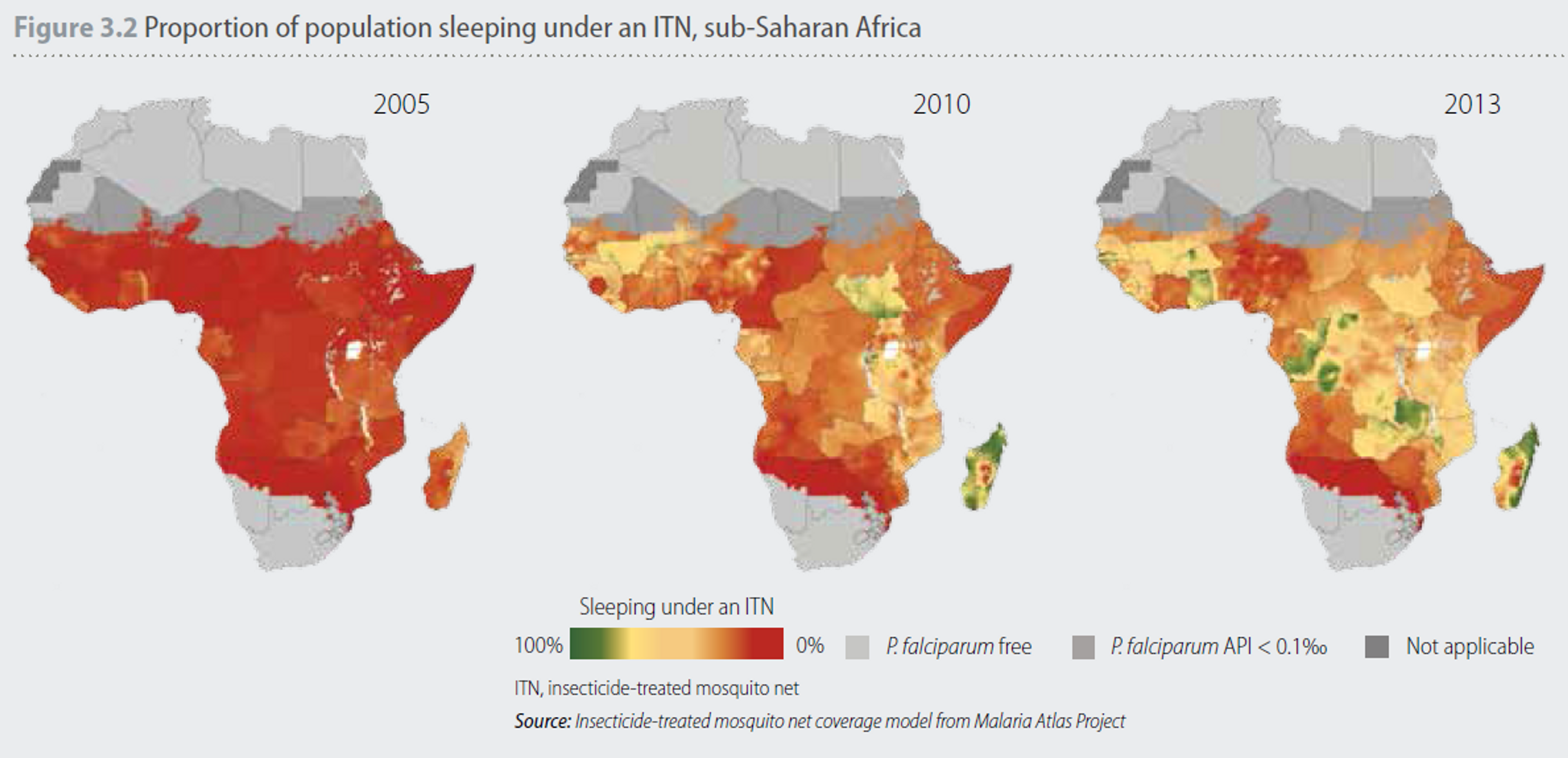
Figure 3: Improvements in access to ITNs and their use vary considerably between different geographical areas. In 2005, the proportion of the population sleeping under an ITN was generally low, with only six countries achieving coverage levels greater than 20%
And even as recently as 2013, 278 million of the 840 million people at risk of malaria lived in households without even a single net[86]. But 2014 has been the strongest year so far with 214 million nets delivered to countries in sub-Saharan Africa (427 million LLINs have been delivered to Africa since 2012 in total[87]. AMF has distributed around 5 million since it has started operations[88]). In 2015, about 200 million nets are already financed[89]. Thus, it is difficult to estimate the true need for nets, but it is likely to be higher than 50 million nets.
In sum, we believe there is substantial room for more funding if AMF scales up its operations and aims for universal access to nets, but think it is conceivable that, because of rapid acceleration of bednet distribution, within the next 10 years AMF’s funding gap might be closed, in which case, it might be difficult for AMF to provide other services.
Updates on Fundraising activities
AMF told Givewell that it is not in conversations with any major funders to help fill its funding gap[90]. They have also been criticized by Givewell for not expanding their small team consisting of only two full time staff that are assisted by volunteers - which Givewell has argued might limit their ability to raise funds as robustly as it potentially could, given AMFs effectiveness. We have talked to AMF about this issue[91] and learned that they are potentially thinking about expanding their team in the near future, but do not need more staff at this point. We think it is good that they have a very lean organisation that is highly specialized and very effective in the area they are working on. We have also learned that AMF does try to raise funds from private donors and I planning to intensify their fundraising efforts in the future. They have also tried to work with an experienced grant writer in the past, but did not find this as effective as other means of raising funds.
Updates on AMF’s Operations
Net distributions
In the DRC (Congo), AMF reports that it has succeeded in the largest distribution of nets to date: 676,000 nets were successfully distributed in the Kasaï Occidental district in November last year[92]. However, another smaller distribution in Congo’s North Idjwi Island of only 62,000 nets has been delayed. AMF has told us[93] that this due to the distribution partner facing delays in carrying out the pre-distribution registration survey (net need per household) upon which the distribution is then based. [94],[95].
In Malawi, a large scale distribution of 396,000 nets was planned in in January-February 2015 and as of April 9th the nets are yet to be delivered[96]. AMF has told us[97] that the nets are in-country but the distribution has been delayed to due to flooding in Malawi (the worst floods in the country for 50 years with at least 174,000 people displaced.) which led to a state of emergency being declared by the government in 15 of the 28 districts in Malawi[98]. AMF tells us that the nets will now be distributed in May[99].
AMF is also considering funding research on investigating insecticide resistance as well as running a pilot project to test the use of smartphone technology for tracking net distributions. We think both research projects are sensible and it is good that AMF continues to improve the evaluation of its interventions to gain better knowledge about them.
Acknowledgements
Thanks to Peter McIntyre, Josh Jacobson and several anonymous reviewers for helpful comments, edits, and feedback on this report.
[1] Abu-Raddad, Laith J, Padmaja Patnaik, and James G Kublin. "Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa." Science 314.5805 (2006): 1603-1606.
[2] Abu-Raddad, Laith J, Padmaja Patnaik, and James G Kublin. "Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa." Science 314.5805 (2006): 1603-1606.
[3] Abu-Raddad, Laith J, Padmaja Patnaik, and James G Kublin. "Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa." Science 314.5805 (2006): 1603-1606.
[4] Barnabas, Ruanne V et al. "The role of co-infections in HIV epidemic trajectory and positive prevention: a systematic review and meta-analysis." AIDS (London, England) 25.13 (2011): 1559.
[5] González, Raquel et al. "HIV and malaria interactions: where do we stand?." 10.2 (2012): 153-165.
[6] Abu-Raddad, Laith J, Padmaja Patnaik, and James G Kublin. "Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa." Science 314.5805 (2006): 1603-1606.
[7] Cuadros, Diego F, Adam J Branscum, and Philip H Crowley. "HIV–malaria co-infection: effects of malaria on the prevalence of HIV in East sub-Saharan Africa." International journal of epidemiology 40.4 (2011): 931-939.
[8] Cuadros, Diego F et al. "Effect of variable transmission rate on the dynamics of HIV in sub-Saharan Africa." BMC infectious diseases 11.1 (2011): 216.
[9] Boily, MC. "Heterosexual risk of HIV-1 infection per sexual act ..." 2009. <http://www.sciencedirect.com/science/article/pii/S1473309909700210>
[10] Cuadros, Diego F et al. "Effect of variable transmission rate on the dynamics of HIV in sub-Saharan Africa." BMC infectious diseases 11.1 (2011): 216.
[11] Abu-Raddad, Laith J et al. "Have the explosive HIV epidemics in sub-Saharan Africa been driven by higher community viral load?." AIDS (London, England) 27.6 (2013): 981.
[12] Baggaley, R. F., & Hollingsworth, T. D. (2015). HIV-1 transmissions during asymptomatic infection: exploring the impact of changes in HIV-1 viral load due to coinfections. Journal of acquired immune deficiency syndromes (1999).
[13] Note that even though the confidence intervals include 0 and cannot rule out that transmission is not affected at all, this does not mean that it is not statistically significant, nor that increased transmission of up to 21 cases can be ruled out
[14] "Breaking DALYs down into YLDs and YLLs for intervention ..." 2015. 20 Apr. 2015 <http://globalprioritiesproject.org/2015/03/ylds-and-ylls/>
[15] Hunsmann, Moritz. "Limits to evidence-based health policymaking: policy hurdles to structural HIV prevention in Tanzania." Social Science & Medicine 74.10 (2012): 1477-1485.
[16] Stillwaggon, Eileen. "Better economic tools for evaluating health and development investments." AIDS 28.3 (2014): 435-437.
[17] Walson, Judd L et al. "Evaluation of impact of long-lasting insecticide-treated bed nets and point-of-use water filters on HIV-1 disease progression in Kenya." Aids 27.9 (2013): 1493-1501.
[18] Kern, Eli et al. "Provision of bednets and water filters to delay HIV‐1 progression: cost‐effectiveness analysis of a Kenyan multisite study." Tropical Medicine & International Health 18.8 (2013): 916-924.
[19] Tay, Sammy CK et al. "The prevalence of malaria among HIV seropositive individuals and the impact of the co-infection on their hemoglobin levels." Annals of clinical microbiology and antimicrobials 14.1 (2015): 10.
[20] Hassani, Ahmed Saadani, and Barbara J Marston. "Impact of Cotrimoxazole and Insecticide-Treated Nets for Malaria Prevention on Key Outcomes Among HIV-Infected Adults in Low-and Middle-Income Countries: A Systematic Review." JAIDS Journal of Acquired Immune Deficiency Syndromes 68 (2015): S306-S317.
[21] Nega, Desalegn et al. "Anemia associated with asymptomatic malaria among pregnant women in the rural surroundings of Arba Minch Town, South Ethiopia." BMC Research Notes 8.1 (2015): 110.
[22] "WHO | Worldwide prevalence of anaemia 1993-2005." 2009. 15 Apr. 2015 <http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/9789241596657/en/>
[23] Gamble, C, John Paul Ekwaru, and Feiko O ter Kuile. "Insecticide-treated nets for preventing malaria in pregnancy." Cochrane Database Syst Rev 2.2 (2006): CD003755.
[24] "PLOS Medicine: Insecticide-Treated Nets for the Prevention ..." 2015. 15 Apr. 2015 <http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.0040107>
[25] van Eijk, Anna Maria et al. "Coverage of intermittent preventive treatment and insecticide-treated nets for the control of malaria during pregnancy in sub-Saharan Africa: a synthesis and meta-analysis of national survey data, 2009–11." The Lancet infectious diseases 13.12 (2013): 1029-1042.
[26] Tay, Sammy CK et al. "The prevalence of malaria among HIV seropositive individuals and the impact of the co-infection on their hemoglobin levels." Annals of clinical microbiology and antimicrobials 14.1 (2015): 10.
[27] Takem, Ebako N, Eric A Achidi, and Peter M Ndumbe. "An update of malaria infection and anaemia in adults in Buea, Cameroon." BMC research notes 3.1 (2010): 121.
[28] Prasittisuk, C. "Vector-control synergies, between'roll back malaria'and the Global Programme to Eliminate Lymphatic Filariasis, in South-east Asia." Annals of tropical medicine and parasitology 96 (2002): S133-7.
[29] Reimer, Lisa J et al. "Insecticidal bed nets and filariasis transmission in Papua New Guinea." New England Journal of Medicine 369.8 (2013): 745-753.
[30] van den Berg, Henk, Louise A Kelly-Hope, and Steve W Lindsay. "Malaria and lymphatic filariasis: the case for integrated vector management." The Lancet infectious diseases 13.1 (2013): 89-94.
[31] van den Berg, Henk, Louise A Kelly-Hope, and Steve W Lindsay. "Malaria and lymphatic filariasis: the case for integrated vector management." The Lancet infectious diseases 13.1 (2013): 89-94.
[32] World Health Organization. "Investing to overcome the global impact of neglected ..." 2015. <http://apps.who.int/iris/bitstream/10665/152781/1/9789241564861_eng.pdf?ua=1>
[33] World Health Organization. "investing to overcome the global impact of neglected ..." 2015. <http://apps.who.int/iris/bitstream/10665/152781/1/9789241564861_eng.pdf?ua=1>
[34] http://www.who.int/neglected_diseases/preventive_chemotherapy/lf/db/?units=minimal®ion=AFR&country=all&countries=all&year=2013
[35] http://www.who.int/neglected_diseases/preventive_chemotherapy/lf/db/?units=minimal®ion=AFR&country=all&countries=all&year=2013
[36] Mzilahowa, Themba et al. "Entomological indices of malaria transmission in Chikhwawa district, Southern Malawi." Malar J 11 (2012): 380.
[37] Nielsen, Nina Odgaard et al. "Co-infection with subclinical HIV and Wuchereria bancrofti, and the role of malaria and hookworms, in adult Tanzanians: infection intensities, CD4/CD8 counts and cytokine responses." Transactions of the Royal Society of Tropical Medicine and Hygiene 101.6 (2007): 602-612.
[38] Baggaley, R. F., & Hollingsworth, T. D. (2015). HIV-1 transmissions during asymptomatic infection: exploring the impact of changes in HIV-1 viral load due to coinfections. Journal of acquired immune deficiency syndromes (1999).
[39] Ayoola, Omolola O et al. "The impact of malaria in pregnancy on changes in blood pressure in children during their first year of life." Hypertension 63.1 (2014): 167-172.
[40] "PLOS Medicine: Ultra-Sensitive Detection of Plasmodium ..." 2015. 11 Apr. 2015 <http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1001788>
[41] "PLOS Medicine: Ultra-Sensitive Detection of Plasmodium ..." 2015. 11 Apr. 2015 <http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1001788>
[42] Murray, Christopher JL et al. "Global malaria mortality between 1980 and 2010: a systematic analysis." The Lancet 379.9814 (2012): 413-431.
[43] Caminade, Cyril et al. "Impact of climate change on global malaria distribution." Proceedings of the National Academy of Sciences 111.9 (2014): 3286-3291.
[44] "WHO | World Malaria Report 2014." 2014. 11 Apr. 2015 <http://www.who.int/malaria/publications/world_malaria_report_2014/en/>
[45] "PLOS Medicine: The Impact of Pyrethroid Resistance on the ..." 2015. 11 Apr. 2015 <http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1001619>
[46] Briet, OJ et al. "Effects of pyrethroid resistance on the cost effectiveness of a mass distribution of long-lasting insecticidal nets: a modelling study." Malar J 12 (2013): 77.
[47] Augustincic Polec, Lana et al. "Strategies to increase the ownership and use of insecticide‐treated bednets to prevent malaria." The Cochrane Library (2014).
[48] Augustincic Polec, Lana et al. "Strategies to increase the ownership and use of insecticide‐treated bednets to prevent malaria." The Cochrane Library (2014).
[49] Orkoh, Emmanuel, and Samuel Kobina Annim. "Source and Use of Insecticide Treated Net and Malaria Prevalence." (2014).
[50] Gingrich, Chris D et al. "Does Free Distribution Of Mosquito Nets Affect Su