Is there a more effective way of getting a (better) malaria vaccine?
World Malaria Day is next week! To celebrate the progress made in the last decade towards eradicating malaria, join our Just One Net campaign and donate a net!
We finally have a 35% effective malaria vaccine1. Last year, the world’s first malaria vaccine, Monsquirix/RTS,S, was recommended by European drugs regulators2 (though there are still some concerns about the safety of the vaccine3) and initial studies of cost-effectiveness have shown positive results, though it is slightly less cost-effective than insecticide treated bednets4. The vaccine has been developed under a product development partnership between the Bill and Melinda Gates Foundation and GSK. In exchange for funding support, GSK have made strong commitments to make the vaccine available at an affordable price5.
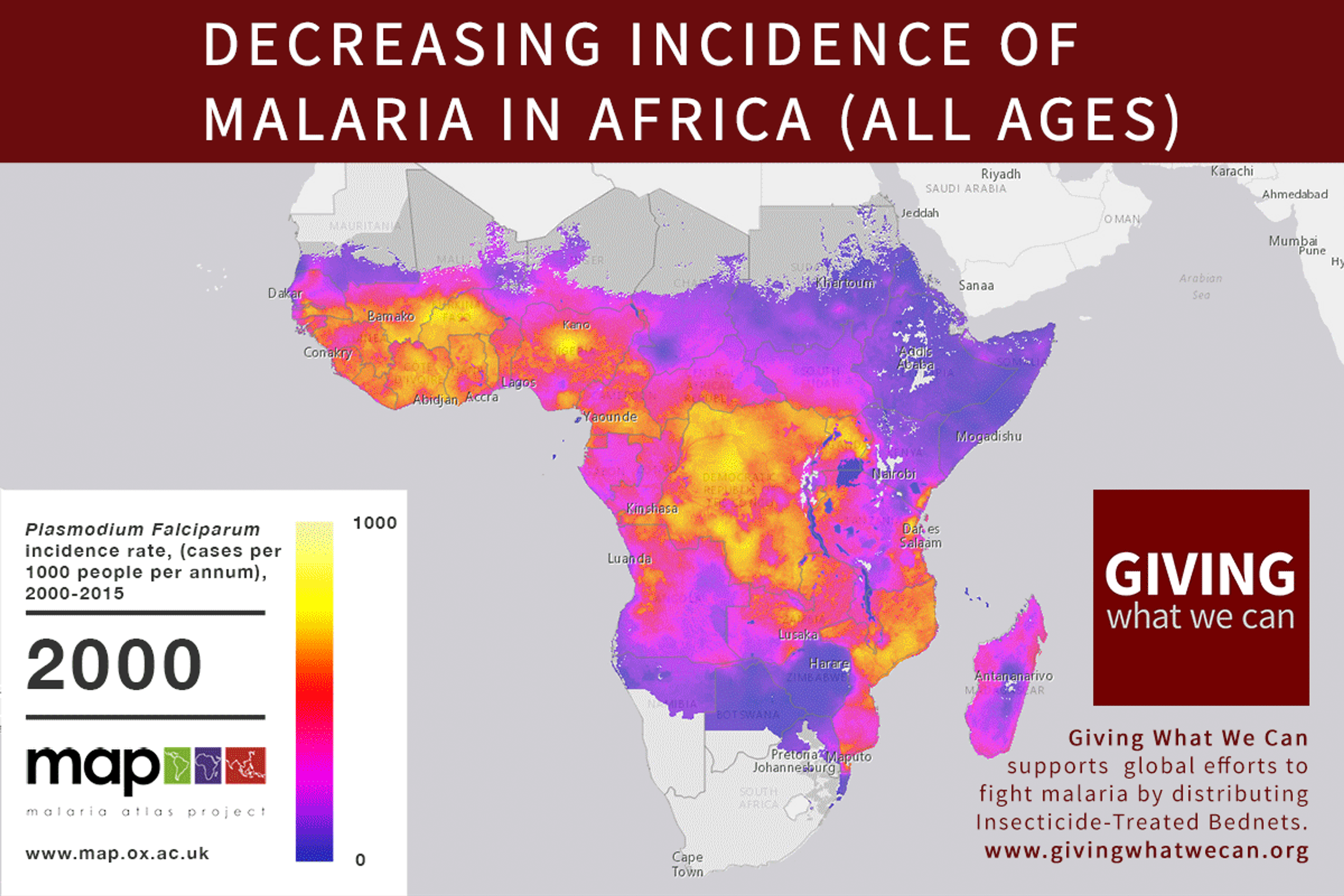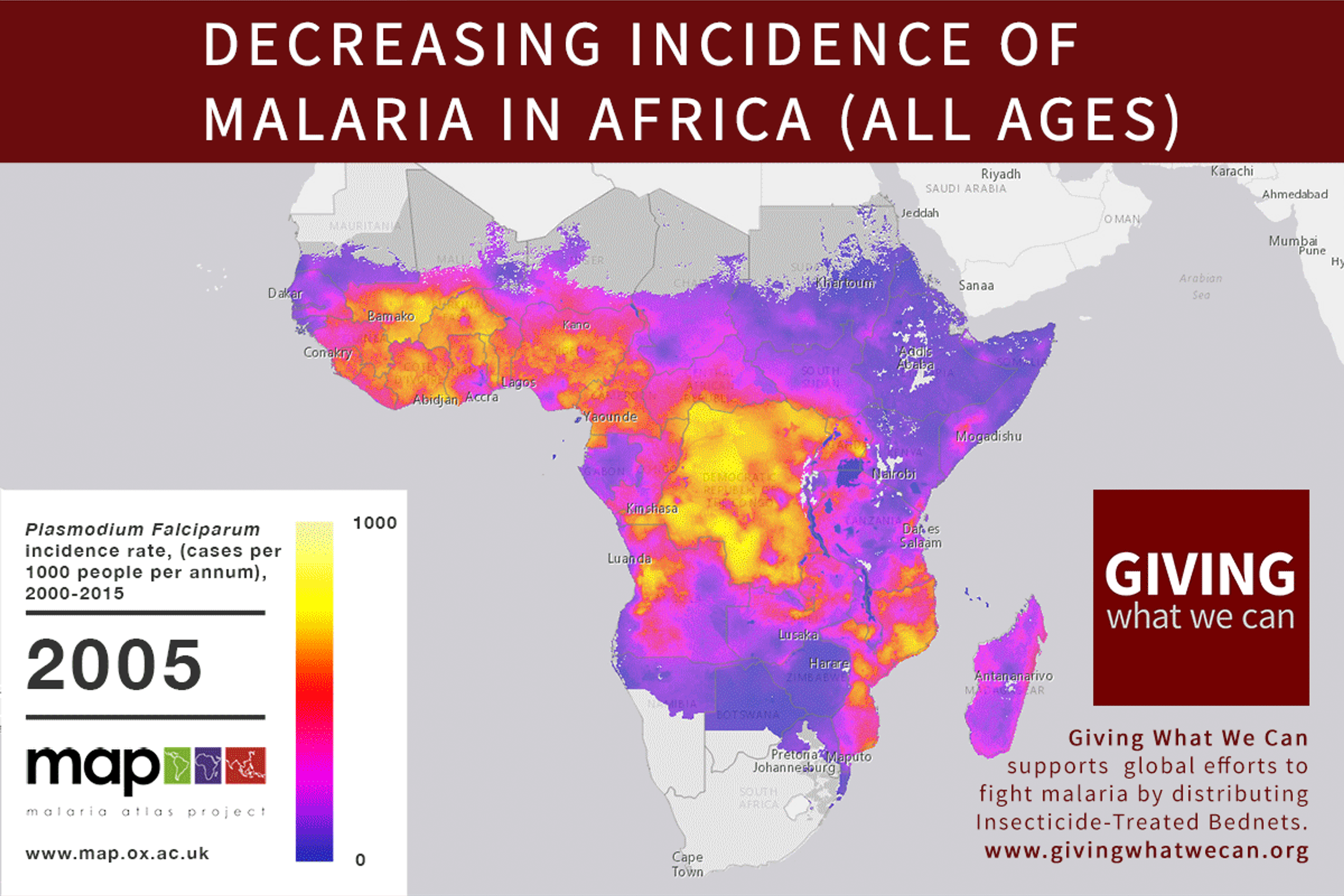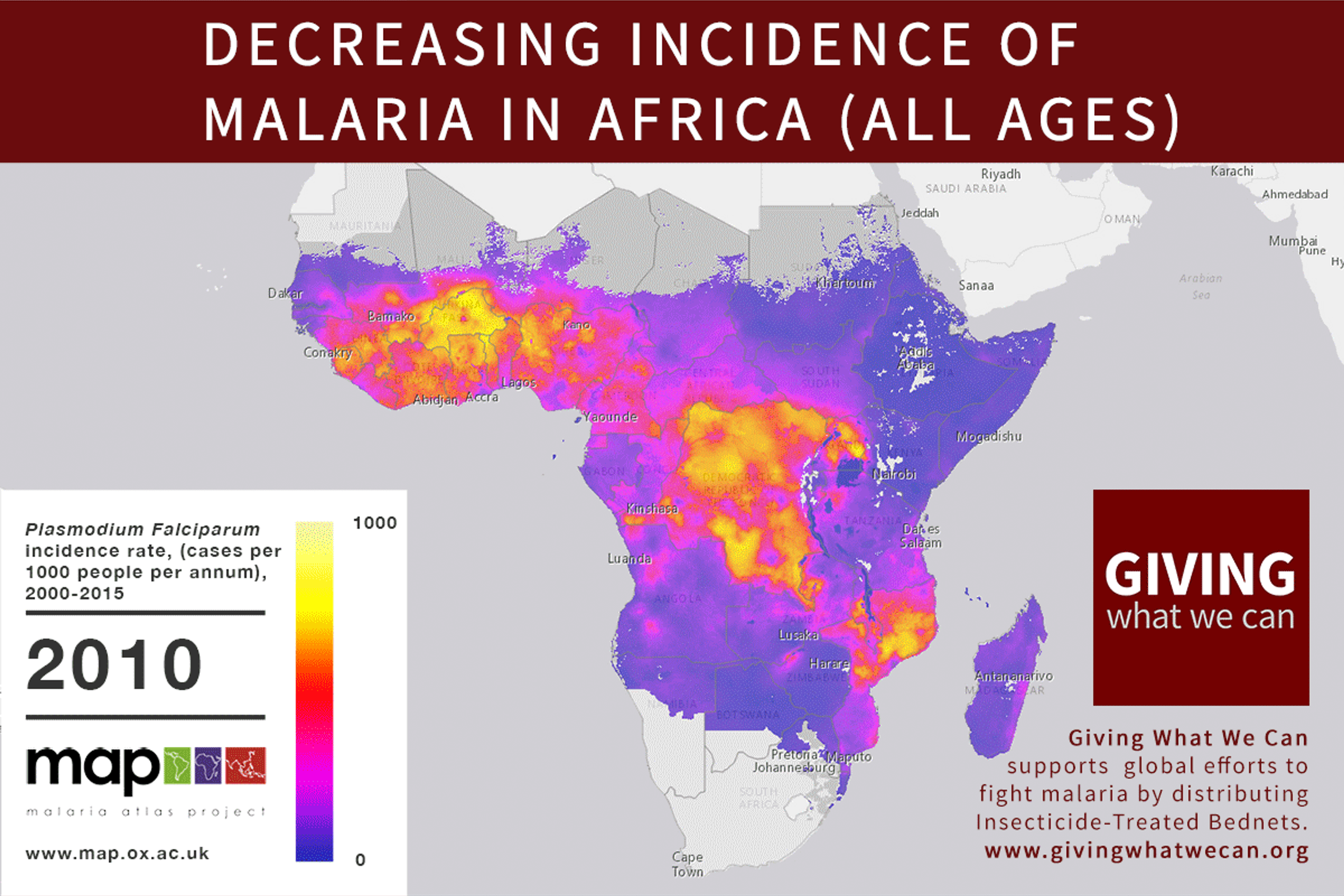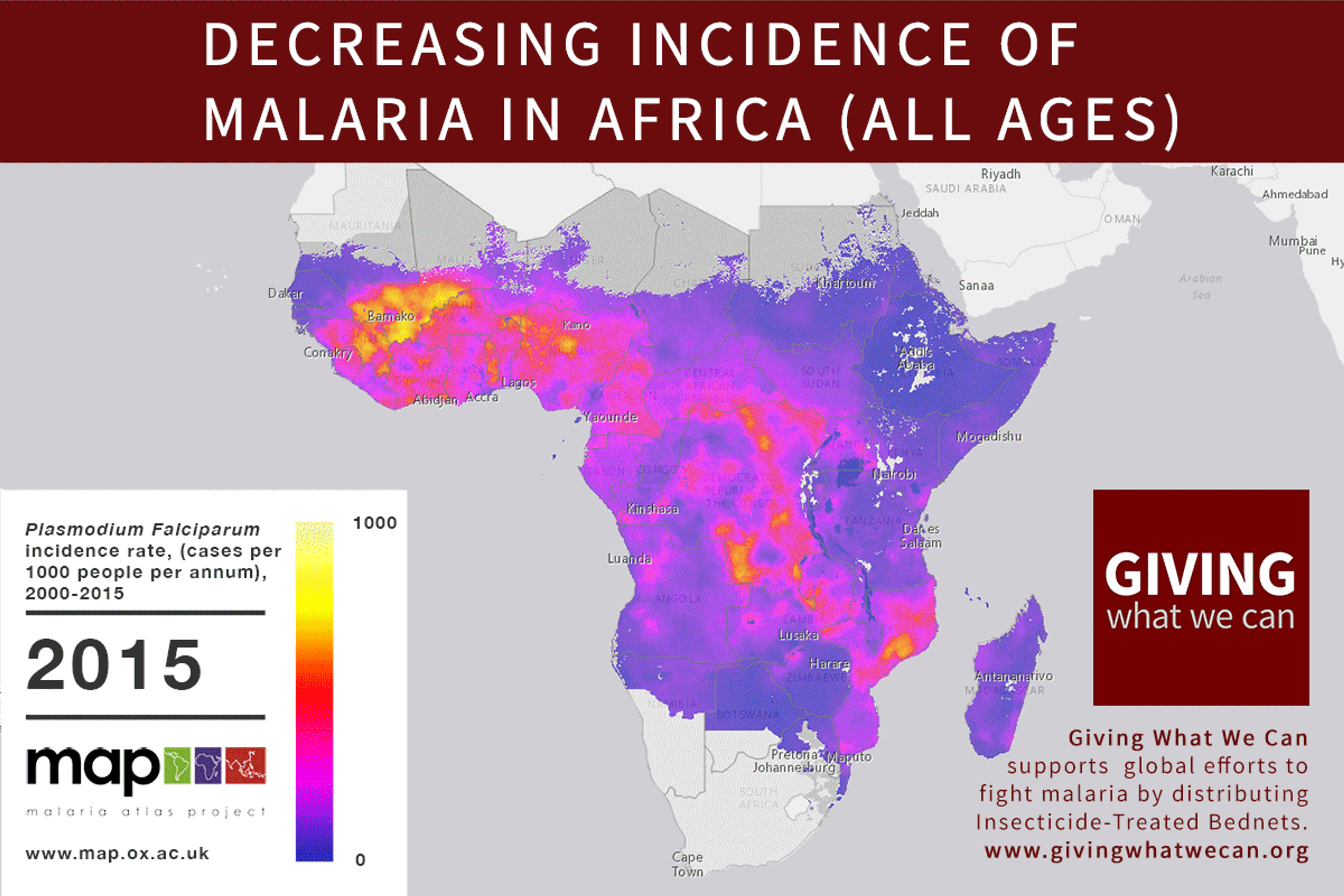
Discovering a new vaccine is excellent news. But we should still question our approach to vaccine development. Is the way we currently fund vaccine and drug research for developing countries working as well as it should? Are we missing out on opportunities? Can we make progress faster?
Because the speed at which we develop a vaccine matters.
Just speeding up the discovery of a vaccine by a single year and the adoption of this vaccine in poor countries by two years, could save many lives4. One analysis suggested that spending $3bn to achieve this would only cost $80 - $90 per additional life year saved6.
So when we think about developing a malaria vaccine we should remember that there are big payoffs if we can speed up the process.
How we fund vaccine development matters
One of the ways we can divide different funding efforts is between push and pull funding mechanisms.
Push funding mechanisms pay for or subsidise research inputs (like paying a team of researchers to do research). Pull funding mechanisms instead pay for research outputs or outcomes, for instance, by paying a prize for successfully developing a drug, or guaranteeing a future price at which a profit can be made7.
In the case of the malaria vaccine, the Gates Foundation pursued a ‘push’ funding approach.
A big downside with push-funding approaches is that they can dis-incentivise other organisations to develop vaccine candidates. In this case, anyone else developing a malaria vaccine would end up competing with a vaccine which will be sold at close to marginal cost8.
Regrettably the RTS,S vaccine offers only modest protection from malaria9: it is about 35% effective, meaning that Mosquirix reduces the number of cases of clinical malaria in children by about 35% over a two year period. This is very low when other highly effective vaccines for children such as polio which is 95% effective.
But there are many of other malaria vaccine candidates being investigated10 - although they are probably receiving much less funding to do so11. So it is perfectly possible there are other more effective vaccines than RTS,S waiting to be developed - and putting money behind just one vaccine, may have slowed or stalled their progress. This is because there is another way of developing vaccines (and indeed other products): Advance Market Commitments. This aims to recreate the market incentives that help develop drugs in richer countries. Donors make legally binding pledges to subsidise vaccines which meet certain requirements. But crucially they aren’t tied to any particular manufacturer. This means that donor organisations don’t have to pay if development is unsuccessful, and they don’t have the difficult decision of assessing which pharmaceutical company has the most promising vaccine prospect. This decision is instead left to pharma companies themselves- who are better placed to evaluate the prospect of success.
This approach was used to develop vaccines for pneumococcal diseases common in the developing world. A collection of governments alongside the Gates Foundation committed $1.5bn as a top-up subsidy for manufacturers if they agreed to develop and supply vaccines at a low price12.
How can we do better in the case of malaria?
Would this approach have worked better in the case of malaria? Would it have found a quicker solution?
Ultimately these are very difficult questions to answer. But these issues of skewed funding incentives were well known over a decade ago13.
There can be downsides to an AMC approach. Critics allege that the AMC could have negotiated a price closer to the cost of production and that other push funding efforts have had more success in negotiating a low price14. The fact that drug companies have voluntarily lowered the ‘tail price’15 under the AMC suggests it was set too high originally. Some see the AMC as overly generous to existing large developed country pharmaceutical companies16.
But almost all the organisations and people involved in vaccine or drug development have strong incentives to prefer push funding. Donors might prefer giving money to support local companies and research outfits. Activist organisations may strongly dislike the idea that private companies can make big profits from pull funding (although they can lose money from unsuccessful research efforts). Drug companies- and also academics- may prefer a guaranteed flow of money for research rather than it being tied to results.
We still have not seen another Advance Market Commitment comparable to that launched for pneumococcal vaccines that was first announced back in 2007. And there are many diseases other than malaria that could benefit from new efforts to develop vaccines or new drugs against them.
So even while we celebrate a new tool against malaria, we should still stop to consider whether there is a better way to get to effective vaccines.